In a hickory its a minor contributor to the overall resident structure and we might want to draw a hybrid that looks something like this. 1 Bond Angles And Shape 2 Lewis Structures 3 Electron Orbitals 4 Polar Bonds Polar Reactions 5 Resonance NW1 Nomenclature Worksheet 1.

Acetamide C2h5no Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic
Learn more about Acetamide structure Properties Production and Uses with Frequently asked Questions here.
. It is the simplest amide derived from acetic acid. Read full article at Wikipedia. Weve got the study and writing resources you need for your assignments.
Draw the Lewis Structure for the ClF2ion and AlF4-. A resonance form is another way of drawing a Lewis dot structure for a given compound. Acetamide CH3CONH2- Acetamide is a chemical compound having the formula CH3CONH2.
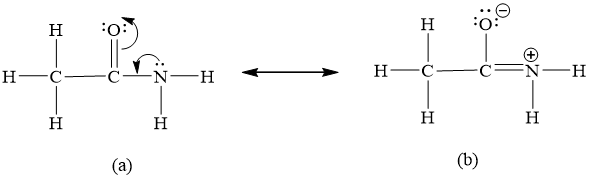
Calculate the of electrons in π bonds pi bonds multiple bonds using formula 1. Draw the lewis structure for resonance forms of acetamide CH3CONH2 Best Answer. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar.
Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar. What resonance structure can account for the planar geometry about the nitrogen atom. Start your trial now.
Draw the Lewis structure for acetamide CH3CONH2 an organic compound and determine the geometry about each interior atom. They are used when there is more than one way to place double bonds and lone pairs on atoms. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar.
Resonance structures arise when there are more than one way to draw a Lewis dot diagram. Where n in this case is 4 since CH3CONH2 consists of nine atoms but five of them is H. Up to 256 cash back Draw the Lewis structure for acetamide CH 3 CONH 2 an organic compound and determine the geometry of each interior atom.
First we need to calculate the total number of valence electrons. Up to 256 cash back Draw the Lewis structure for the acetamide CH3CONH2 an organic compound and determine the geometry about each interior atom. Acetamide is the simplest amide.
Expert Solution Want to see the full answer. Draw the Lewis structure for acetamide CH 3 CONH 2 an organic compound and determine the geometry about each interior atomExperiments show that the geometry about the nitrogen atom in acetamide is nearly planar. C H 3 C O N H 2 mathrm CH_3CONH_2 C H 3 CON H 2.
What resonance structure can account for the planar geometry about the nitrogen atom. Which resonance structure can account for the planar geometry about the nitrogen atom. Resonance hybridization lewis structures orbitals.
To draw the Lewis structure first draw the bond skeleton. What resonance structure can account for the planar geometry of the nitrogen atom. This problem has been solved.
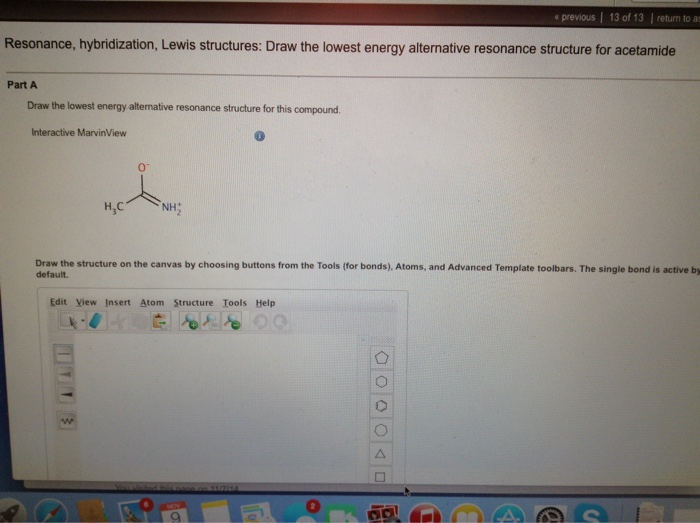
Lets write Lewis structure for acetamide. Equivalent Lewis structures are called resonance forms. Draw the molecule by placing atoms on the grid and connecting them with bonds.
1 5 4 2 6 5 24 1 cdot 5 4 cdot 2 6 5 24 1 5 4 2 6 5 24. Solution for Draw a second resonance structure for acetamide. These are the electron dot structure which shows the bonding between the atoms of the molecule and lone pairs of electronif present on an atom Resonance structures.
Draw the Lewis structure for acetamide CH3CONH2 an organic compound and determine the geometry about each interior atom. First week only 499. So even though this isnt a great resonance structure it does exist too.
Visit BYJUS for more content. It is a tautomer of an acetimidic acid. It is derived from acetic acid.
Acetamide is a member of the class of acetamides that results from the formal condensation of acetic acid with ammonia. This is the best answer based on feedback and ratings. Draw the Lewis structure for acetamide CH3CONH2 an organic compound and determine the geometry about each interior atom.
When you account for the bond pairs there are 12 remaining electrons to distribute. See the answer See the answer done loading. It is a monocarboxylic acid amide a N-acylammonia and a member of acetamides.
We know that resident structures are really just ah hybrid or compounds are really just a hybrid of the residence forms. Although the notation may be confusing the C in the center of the molecule is bound to a group an O and an group. Experiments show that the geometry of the nitrogen atom in acetamide is nearly planar.
Hydrogen has 1 valence electron carbon has 4 valence electrons oxygen has 6 valence electrons nitrogen has 5 valence electrons. It is used as a plasticizer hygroscopic agent and fire suppressant. Step 1 of 3.
Ethanamide is an organic compound with the formula CH 3 CONH 2. It finds some use as a plasticizer and as an industrial solvent. Connect the atoms of acetamide with single bonds.
These are the structure which shows the delocalizing of electrons in a moleculeWhen the molecule have more than one Lewis structure those structure are known. Naming Alkanes Cycloalkanes 6 Alkanes Alkenes 7 Cycloalkanes NW2 Nomenclature.

Solved Draw The Lewis Structure For Resonance Forms Of Chegg Com

Solved Draw The Lewis Structure For Acetamide Left Mathrm Ch 3 Mathrm Conh 2 Right An Organic Compound And Determine The Geometry About Each Interior Atom Experiments Show That The Geometry About The Nitrogen Atom In Acetamide Is Nearly
Solved Resonance Hybridization Lewis Structures Draw The Chegg Com
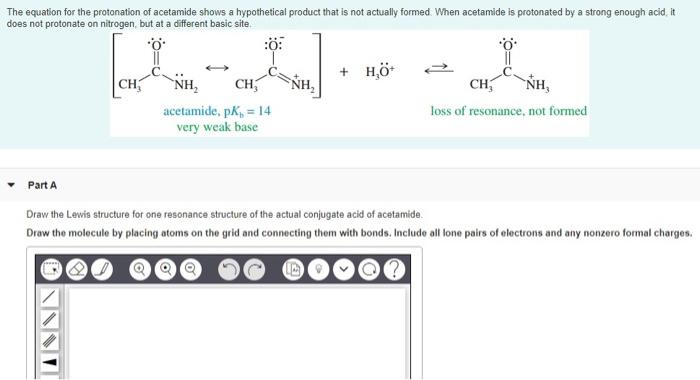
Solved The Equation For The Protonation Of Acetamide Shows A Chegg Com
Solved Draw The Lewis Structure For Acetamide Left Mathrm Ch 3 Mathrm Conh 2 Right An Organic Compound And Determine The Geometry About Each Interior Atom Experiments Show That The Geometry About The Nitrogen Atom In Acetamide Is Nearly

Chemistry Net Construct The Electron Dot Structure Of Acetamide Electrons Dots Pi Bond

Lewis Configuration Of The Allylic Carbocation Chemistry Lewis Configuration

Solved The Amide Group In Acetamide As Well As In All Other Amid Chegg Com
0 comments
Post a Comment